

POJ 17(01):10-13 (2025) | ISSN:1836-3644
doi: 10.21475/POJ.17.01.25.p13
Seed quality of white oats with different foliar applications of zinc
Marta Gubert Tremea¹, Guilherme Roberto Schalanski¹, Gerusa Massuquini Conceição², José Antonio Gonzalez da Silva², Maria Eduarda Schmidt², Cibele Luisa Peter², Laura Eduarda Arnold², Joeli Vaz Bagolin²
¹Federal University de Pelotas, Brasil
²Northwest Regional University of the State of Rio Grande do Sul, Brasil
Abstract: The objective of this study was to evaluate the physiological quality of white oat seeds produced through foliar biofortification with zinc. The experiment was conducted in the experimental area of the Regional Institute for Rural Development (IRDeR), where white oats were sown and biofortified with zinc at doses of 0, 1000, 2000, and 4000 g ha⁻¹, applied at two different physiological stages of the plants, constituting a completely randomized experimental design with two factors. The analyzed variables were seed yield, thousand-seed weight, first germination count, germination, accelerated aging, seedling length, seedling dry mass, and electrical conductivity. The application of zinc through foliar biofortification only influenced yield when more than one application was performed, resulting in above-average outcomes, i.e., greater than 1600 kg ha⁻¹. For seed quality, the number of zinc applications did not differ statistically; however, different zinc doses had a significant influence on the first germination count, germination, accelerated aging, and seedling length tests, as well as on yield components, demonstrating the effectiveness of using foliar zinc biofortification for both the quantity and quality of white oat seed production.
Keywords: Avena sativa; Biofortification; Vigor; Germination; Physiology.
Introduction
Micronutrient deficiencies affect the cultivation of various crops, including white oats, which is a grass with high productive potential and is increasingly expanding its cultivation area in Brazil. In this context, to ensure quality and quantity in oat production, several factors are essential, such as effective management in fertilization practices and the use of high-quality seeds to guarantee the establishment and development of the crop in the field (Machado and Silveira, 2020).
The proper development of seedlings stems from the physiological quality of the seeds sown in the field, primarily characterized by high germination rates, vigor, and chemical composition, which are enhanced by the availability of nutrients that promote plant growth and development (Paulilo et al., 2015; Wenneck et al., 2020), ultimately leading to high productive ceilings. In this regard, a deficiency or excess of nutrients can cause imbalances, resulting in reduced growth or even death by hindering fundamental physiological processes essential for the enzymatic system involved in photosynthesis and respiration (Conceição, 2016; Finoto et al., 2020).
Foliar biofortification with zinc is a technique that promotes significant changes in the expression of productivity and quality of the produced seeds, as this micronutrient is directly related to protein synthesis, hormone regulation, energy synthesis, and acts as a cofactor for enzymes that play a fundamental role in the optimal metabolic functioning of plants (Oshe and Santos, 2020; Melo et al., 2021). Therefore, the objective of this study was to evaluate the physiological quality of white oat seeds produced through foliar biofortification with zinc.
Results and Discussion
Based on the obtained data, it was possible to identify that performing two foliar applications of zinc influences the seed yield of white oats; however, it does not affect the physiological quality of the produced seeds, both in terms of germination and vigor tests. In addition to the number of applications of the micronutrient, the different doses influenced the quality of white oat seeds in the first germination count, germination, accelerated aging, and seedling length tests, as well as on the yield components. The interaction between the number of biofortification applications and micronutrient doses followed the same trend.
This highlights the importance of zinc application for the development of this grass due to its involvement in the metabolic reactions of plants, which ensures better growth and functioning, thereby securing satisfactory results for the production of quality seeds.
Based on the regression analysis of the significant variables, the seed yield of white oats (Figure 1) decreases with the application of foliar biofortification with zinc and also with increasing doses, indicating that this micronutrient is not advantageous for enhancing productivity. For the thousand-seed weight (Figure 1), the application of zinc only at the beginning of grain filling was more promising than performing two applications during this period. Furthermore, increasing the dose of zinc applied at the beginning of grain filling resulted in a greater seed mass.
The First Germination Count (Figure 2) showed that applications at two different times during the seed filling of oats do not influence vigor; however, the different doses result in increased vigor as the dose increases. The germination variable (Figure 2) follows the same trend as the First Germination Count. When foliar biofortification with zinc was applied at the beginning of grain filling, the use of a dose of 2000 g/ha resulted in higher physiological quality of the seeds. Different results were found by Consoli (2023), where increasing the zinc dose reduced the germination of black oat seeds; similarly, Yagi et al. (2006), using sorghum seeds treated with different zinc doses, found a reduction in germination percentage as the doses increased.
For the Accelerated Aging test (Figure 2), regardless of whether zinc was applied only at the beginning of grain filling or with two applications during filling, the zinc doses reduced seed vigor. Thus, it becomes viable not to perform foliar biofortification with zinc, irrespective of any applications made during grain filling.
Table 1. Root mean square of the variables productivity (PROD), thousand seed weight (MMS), first germination count (PCG), germination (G), accelerated aging (EA), root length (CR), shoot length (CPA), total length (CT), seedling dry weight (MSP) and electrical conductivity (CE).
| FV | G | PROD | MMG | PCG | G | EA | CR | CPA | CT | MSP | CE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N. ap | 1 | 177928.22* | 0.62 | 7.03 | 7.03 | 30.03 | 0.87 | 2.66 | 0.50 | 0.002 | 66.12 |
| Dose | 3 | 146999.28* | 25.29* | 38.69* | 38.69* | 40.61* | 9.96* | 3.44* | 20.94* | 0.12 | 268.20 |
| N.*Dose | 3 | 33759.24 | 16.20* | 43.61* | 43.61* | 4.36 | 25.75* | 7.87* | 57.49* | 0.02 | 236.20 |
| Erro | 24 | 14849.02 | 0.80 | 10.07 | 10.07 | 12.15 | 0.99 | 0.68 | 2.19 | 0.01 | 148.14 |
| CV (%) | 7.63 | 2.76 | 3.50 | 3.50 | 3.84 | 5.21 | 6.18 | 4.54 | 22.00 | 7.15 | |
| Média | 1597.41 | 32.40 | 90.59 | 90.59 | 90.90 | 19.17 | 13.42 | 32.59 | 51.6 | 170.18 |
* Significant by Tukey's test when p<0.05.
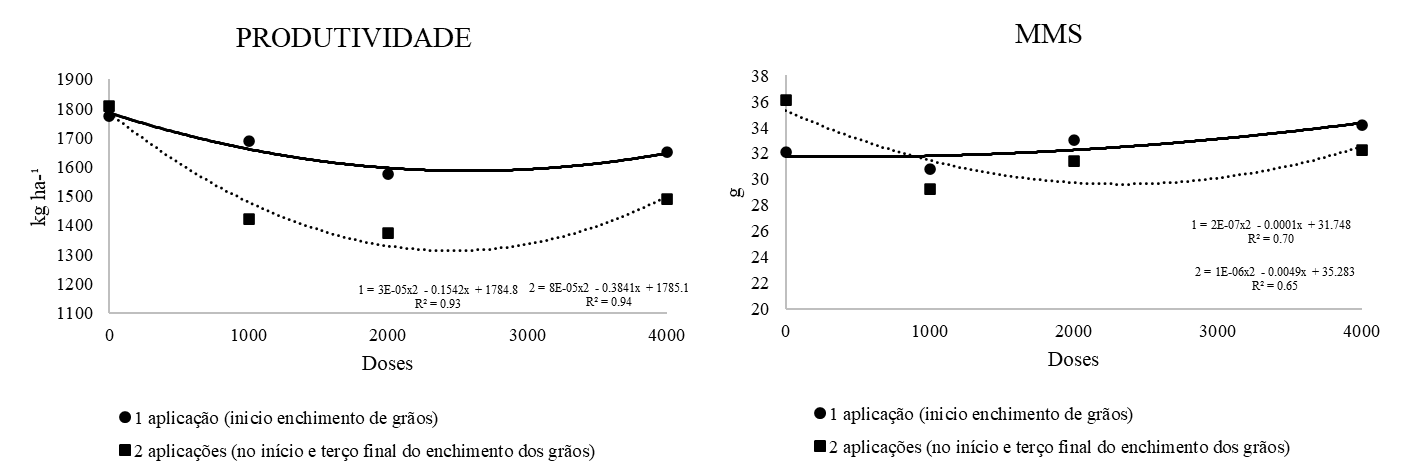
Figure 1. Productivity (productivity) and thousand seed mass (MMS) of white oats produced with zinc applied via foliar application.
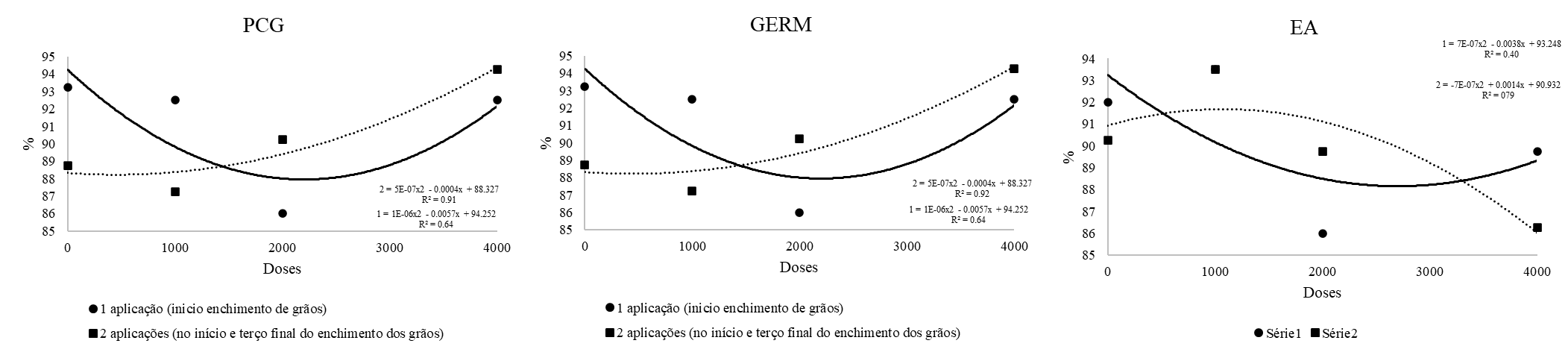
Figure 2. First germination count (PCG), germination (G) and accelerated aging (EA) of white oat seeds produced with foliar-applied zinc.
For Root Length (Figure 3), the use of zinc with one or two applications was promising when using doses of 1000 and 2000 g/ha, reducing vigor with doses starting at 2000 g/ha. However, Consoli (2023) obtained better results without the use of zinc. Baran (2013) demonstrated in his study that increases from 0 to 750 mg of zinc could reduce root size by up to 29.2%. For Total Length (Figure 3), it follows the same trend as Root Length.
For Shoot Length (Figure 3), only one application of biofortification influenced the length, increasing with the applied dose. In contrast, when two foliar biofortifications with zinc were applied to white oats, the behavior remained stable at any dose. Therefore, the ideal approach for shoot length is to apply 2000 g/ha of zinc only at the beginning of grain filling.
The accumulation of zinc in the roots is associated with a decrease in the percentage of dry matter; however, this does not indicate the plant's tolerance to toxic levels of Zn in the substrate (Longnecker and Robson, 1993). Consequently, it ultimately affects root growth when the plant comes into contact with the element at toxic levels.
Materials and methods
Location and experiment description
The present study was conducted in the experimental area of the Regional Institute for Rural Development (IRDeR) in the municipality of Augusto Pestana/RS, geographically located at latitudes 28° 26' 30'' S and longitude 54° 00' 58'' W, and at the Seed Production and Technology Analysis and Research Laboratory at UNIJUÍ.
The sowing of white oats was performed in the second week of June 2022, using the cultivar URS Taura. The experimental units consisted of 5 rows, 5 m in length, with a spacing of 0.20 m between rows. The sowing density used was 300 seeds per square meter. Fertilization was carried out based on soil analysis, aiming for a grain yield of 3 t ha⁻¹. At the time of sowing, 60 and 50 kg ha⁻¹ of P2O5 and K2O were applied, respectively. Regarding nitrogen fertilization, 10 kg ha⁻¹ was applied at sowing, and the remainder was covered in the form of 45% urea when the plants were at the phenological stage indicated by the fourth expanded leaf (V4) according to Zadoks et al. (1974).

Figure 3. Root length (RL), shoot length (CPA) and total length (TL) of white oat seeds produced with foliar-applied zinc.
Experimental design
In the field, a large-scale experiment was conducted using a randomized block design with 4 replications in a 4x2 factorial scheme for four zinc doses (0, 1000, 2000, and 4000 g ha⁻¹), applied either as a single application (at the beginning of grain filling) or sequentially (at the beginning and the final third of grain filling), respectively. The applications were carried out with a backpack sprayer, using a spray volume of 500 liters ha⁻¹.
The experiment was harvested 130 days after emergence, at the full maturity stage, in phenological stage 90, according to Zadoks
(1974). The harvest was mechanized, cutting the three central rows of each plot. After collection, the samples were cleaned, moisture was checked, and then stored in the Seed Laboratory at UNIJUÍ.
The seed moisture was around 12%. In the laboratory, the experimental design was completely randomized, with 4 replications.
Analyzed variables
To estimate seed yield, the seed mass from the harvest of the three central rows of each plot was used and subsequently weighed on a precision scale. The thousand-seed weight (TSW) was determined by counting 250 seeds, which were weighed and then converted to 1000 seeds.
To assess the physiological quality of the seeds, the following tests were conducted: First germination count and germination:
Performed according to the Rules for Seed Analysis (RAS) (Brazil, 2009). The results were expressed as a percentage of normal seedlings.
Accelerated Aging: The seeds were suspended in an aluminum mesh and placed inside a gerbox with 40 mL of distilled water, then subjected to a temperature of 40ºC for 48 hours. Following this, the germination test was conducted according to the methodology described by the RAS (2009). The results were expressed as a percentage of normal seedlings.
Seedling Length: The length of 15 normal seedlings was randomly assessed, obtained from sowing in the upper third of filter paper. The paper rolls containing the seeds were kept in a germinator at a temperature of 20ºC for seven days, after which the lengths of the shoot, root, and total length were measured. The results were expressed in centimeters.
Dry Mass of Seedlings: The seedlings resulting from the length assessment were placed in an oven at 60ºC for 96 hours, housed in paper bags. They were then weighed on a precision scale to determine the total dry mass of normal seedlings, expressed in g/seedling.
Electrical Conductivity Test: Four replications of 25 seeds with seed coats were immersed in 25 mL of distilled water and maintained in a BOD chamber at 20°C for 24 hours. The electrical conductivity of the solution was determined using readings from an AZ-86555 pH/ORP/Cond./TDS/Salinity meter.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) with a 5% error probability. For the variables that showed significant effects, a mean test was performed for qualitative sources of variation, and polynomial regression was conducted for quantitative sources of variation with a 5% error probability.
The statistical analyses were performed using the free software GENES. Non-significant linear or quadratic functions were not presented in the results, even though they were significant according to the ANOVA test.
Conclusion
The application of zinc through foliar biofortification only influences productivity when more than one application is made.
For seed quality, two applications of zinc do not show significant differences. However, different doses of micronutrients influenced the quality of white oat seeds in the first germination count, germination, accelerated aging, seedling length, and productivity components
References
Baran A (2013) Avaliação da sensibilidade de Zea mays ao conteúdo tóxico de zinco no solo. Revista Polonesa de Estudos Ambientais, 22 (1):77-83.
Barros, JFC (2020) Fertilidade do solo e nutrição de plantas. Évora: 5-8.
Brasil (2009) Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes/Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. – Brasília: 339.
Conceição GM, Schiavo J (2020). QUALIDADE DE SEMENTES DE AVEIA BRANCA: um complexo de atributos interagindo nos diferentes aspectos do desempenho. In: Silva JAZ, Carvalho IR, Magano DM. A CULTURA DA AVEIA da semente ao sabor de uma espécie multifuncional. CRV (Ed) 1(13): 257.
Consoli TC (2023). Germinação de sementes e crescimento de plântulas de aveia preta (Avena strigosa Schreb.) submetidas a diferentes concentrações de zinco. Trabalho de Conclusão de Curso- TCC:UFFC.
Finoto Rasteiro BA; Zufi Junior, FC, Fischer Filho, JA (2020) Interação de nitrogênio e zinco na produção de milho. Ciência & Tecnologia, 12(1): 97–109.
Longnecker NE, Robson AD (1993). Distribuição e transporte de zinco em plantas. Em: Robson AD (ed) Zinco em solos e plantas. Dordrecht: Kluwer Academic.
Machado JM, Silveira DC (2020) Importancia da aveia branca na alimentação humana e animal. Em: Silva JAG, Carvalho IR, Magano DA. A cultura da aveia branca: da semente ao sabor de uma espécie multifuncional, 3ª ed. Ijui, RS.
Mello WM, Santos JO, Oshe S (2021) Vigor de sementes de milho tratadas com bioestimulantes. Visão Acadêmica. 22(1).
Mendes MLM et al (2016) Hábitos alimentares e atividade física de universitários da área de saúde do município de Petrolina-PE. Actas de Saúde Colet. 10(2):205-217.
Paulilo, MTS, Viana AM, Randi AM (2015) Fisiologia Vegetal. Florianópolis: Universidade Federal de Santa Catarina: 182.
Ohse S, Pinheiro dos Santos LL (2020) Modos de aplicação de zinco em genótipos de milho. Revista Campo Digital, 15(1).
Wenneck GS, Saath R, Volpato CS, Araujo LL, Sa NO, Santi DC (2020) Nutrientes em sementes de soja em função da aplicação de zinco. Acta Iguazu, 9(3):20–27.
Yagi R et al (2006) Aplicação de zinco via sementes e seu efeito na germinação, nutrição e desenvolvimento inicial do sorgo. Pesquisa Agropecuária Brasileira, 41(4):655-660.
Zadoks JC, Chang TT, Konzak CF (1974) Um código decimal para os estágios de crescimento dos cereais. Pesquisa de Ervas Daninhas. 14:415-421.